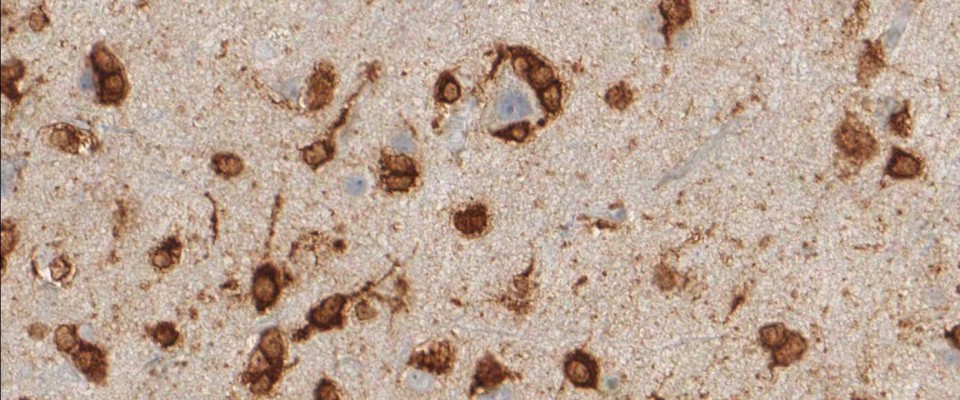
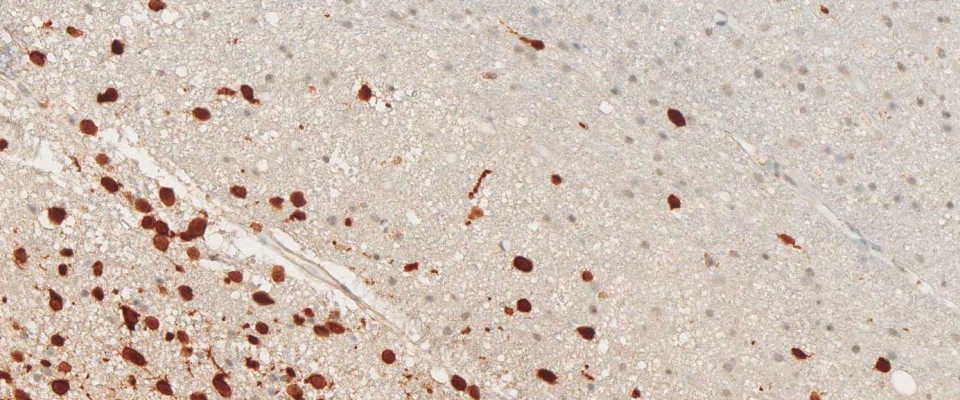
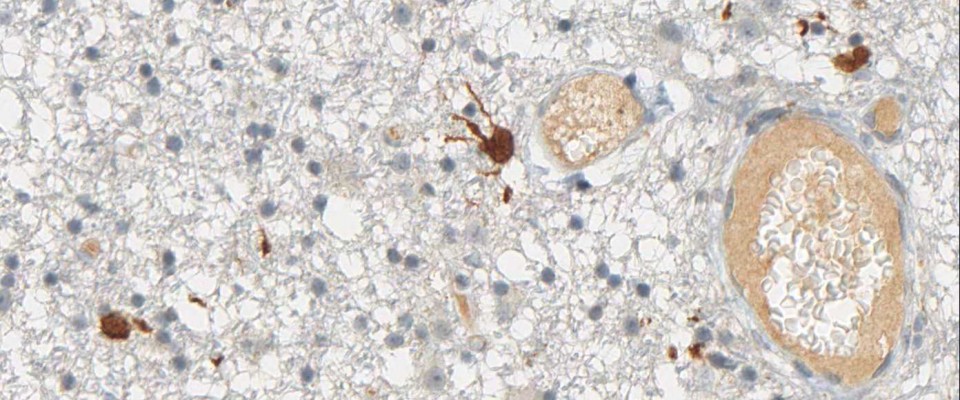
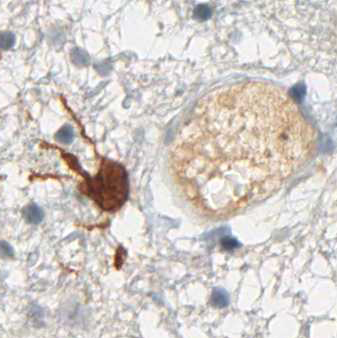
More than 70% of all Gliomas exhibit the IDH1 R132H point mutation. No other type of tumor displays the same mutation with such frequency. The mutant protein can reliably be detected using a highly specific antibody (clone H09) developed at the Department of Neuropathology together with the Clinical Cooperation Unit Neuropathology at the German Cancer Research Center in Heidelberg, Germany. The high frequency of IDH1 R132H mutation in lowgrade and anaplastic gliomas and secondary glioblastomas correlates with favorable patient survival times. Rational IDH1 R132H testing supports neuropathological differential diagnosis and provides valuable prognostic information for the treating.
The WHO-classification for CNS Tumors 2016 recommends the immunohistochemical determination of IDH1 R132H on formalin-fixed tissues as an initial step in glioma diagnosis. Successive integration of IHC procedures reduces the number of molecular tests required for unequivocal diagnosis (Reus et al., Acta Neuropathol. 129, 2015).
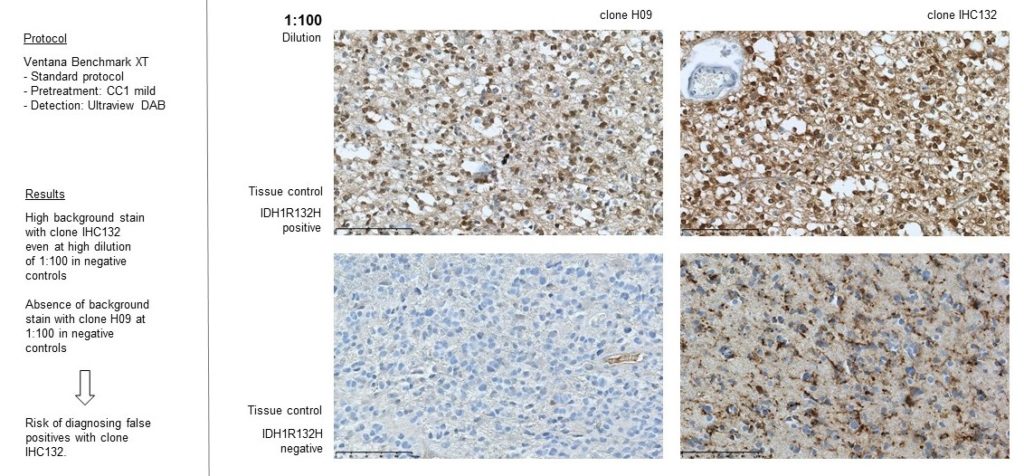
Clone H09 is the goldstandard for IDH1 R132H detection in glioma
Download handout – Competitor study
Diagnostic Applications
IDH1 R132H immunohistochemistry has changed routine diagnostic neuropathology, because it allows narrowing down the possible diagnosis to the group of diffusely infiltrating gliomas of WHO Grades II and III and secondary glioblastoma and to a certain extent to primary glioblastoma. The determination of the IDH1 mutation status strongly supports the differential diagnosis between an anaplastic glioma and a glioblastoma. Furthermore, the detection of even single IDH1 R132H-positive cells clearly supports the diagnosis of a diffusely infiltrating glioma. Again, the detection of positive cells by IDH1 R132H immunohistochemistry allows a clear and safe separation between low-grade glioma and reactive gliosis.
Prognostic Implications
The presence of IDH mutations in diffuse gliomas has been shown to be associated with favorable patient survival time in several studies. This effect seems to be independent from other strong prognostic factors and correlated with 1p/19q deletion and MGMT promoter methylation. In studies pooling low-grade astrocytomas and oligodendrogliomas the IDH mutation status was prognostic for overall and progression free survival. In primary glioblastoma IDH1 mutational status has been reported to be the only factor that showed significant association with patient survival times. The consistent finding of a more favorable outcome of diffuse glioma patients with IDH mutations implies that IDH testing might be useful for prognostic considerations in the clinical setting.
Predictive Value
IDH mutations were correlated with a higher rate of responses to up-front temozolomide in low-grade glioma patients. Also, evidence for differential responsiveness to genotoxic therapy of IDH1 mutant versus IDH1 wild-type lowgrade gliomas has been provided: The presence of IDH1 mutations was associated with favorable progression-free and overall survival in WHO Grade II gliomas who received radio- or chemotherapy. Current studies aim to validate and clarify the predictive value of IDH mutations in the different glioma types. Moreover, it remains to be seen whether targeted therapies may be effective in IDH-mutant gliomas. In 2014 cancer researchers from Heidelberg reported first positive results toward the therapeutic application of a IDH1 R132H mutation-specific vaccine.