IDH mutations in glioblastoma and low-grade glioma
In 2008 whole genome analysis of human glioblastomas led to the discovery of mutations in the active site of isocitrate dehydrogenase 1 (IDH1). Initially, IDH1 mutations affecting the highly conserved arginine (R) residue at codon 132 were found with a frequeny of 12% in WHO grade IV glioblastoma. They occurred especially in secondary glioblastoma and were associated with a significant increase in overall patient survival.
After the discovery of IDH mutations in secondary glioblastoma studies were conducted to verify presence of these mutations in low grade gliomas: Approximately 80% of all WHO grade II–III infiltrating/diffuse gliomas (astrocytomas, oligodendrogliomas,and oligoastrocytomas) and secondary glioblastomas show mutations in IDH1 and to a lesser extent IDH2. In contrast to diffuse gliomas, IDH mutations are very rare or absent in a variety of WHO grade I and II CNS tumors, such as pilocytic astrocytomas, subependymal giant cell tumors, gangliogliomas, ependymomas and pleiomorphic xanthoastrocytomas.
Mutations in IDH1 and IDH2 are considered as strong prognostic marker, independently from the other well-known prognostic factors, and correlate with enhanced patient survival. By far the most common mutation is IDH1 R132, it occurs in roughly 70% of astrocytomas and oligodendroglial tumors
In contrast to secondary glioblastoma, IDH1 or IDH2 mutations were only found in a minority of primary glioblastoma (<5-10%). IDH mutations were only rarely identified in pediatric patients (approximately 16%).
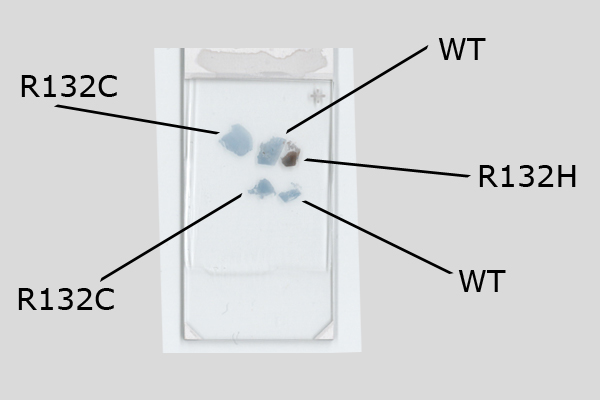
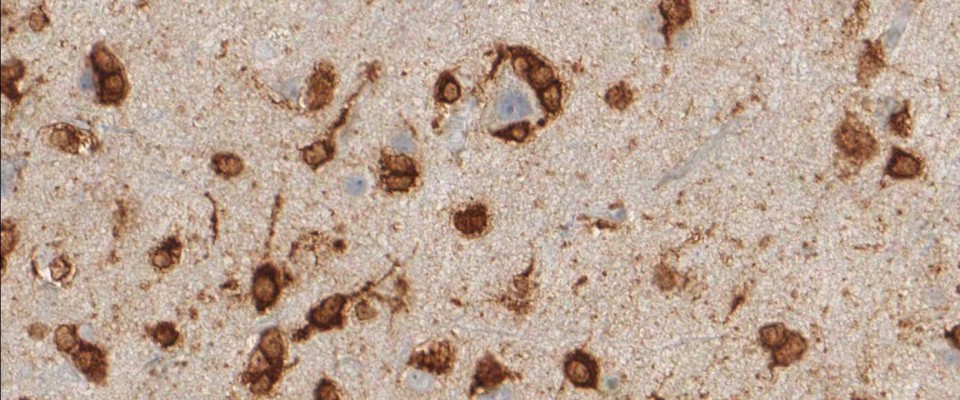
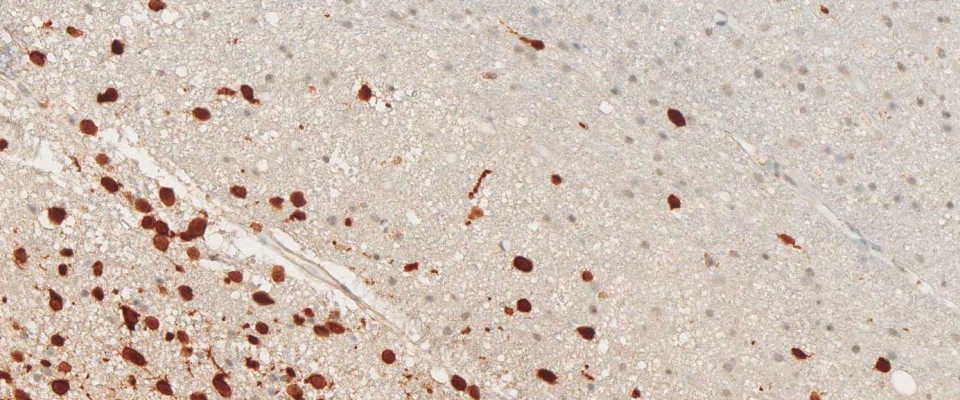
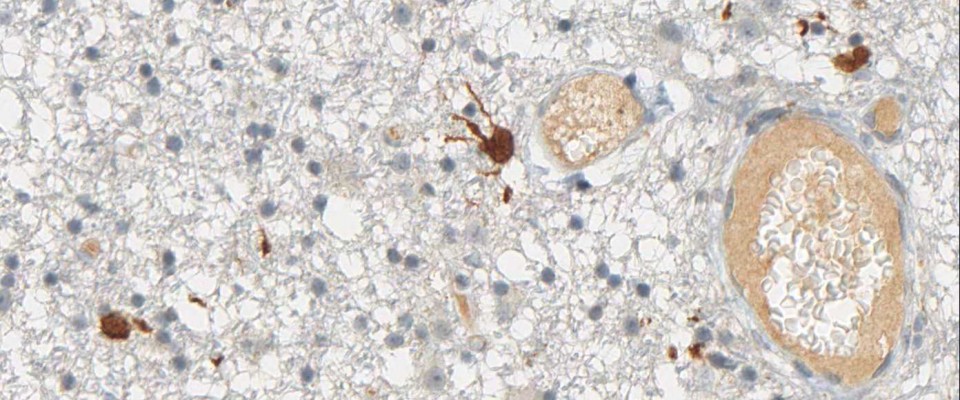
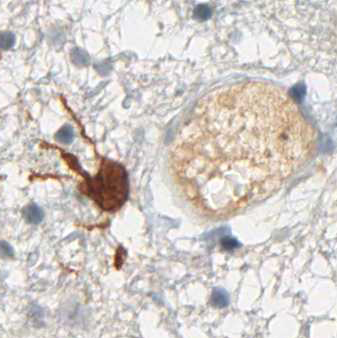
Binding Characteristics of antibody clone H09 in a tissue array (FFPE) with brain tumor sections of different IDH1 status. IDH1 R132H, oligiodendroglioma WHO II, IDH1 R132C, astrocytoma WHO II IDH1 wild type, glioblastoma. Monoclonal antibody clone H09 specifically and exclusively detects the R132H point mutated IDH1, but does not bind to the unaltered enzyme (IDH1 wild type) nor binds to the IDH1 R132C mutated protein (picture courtesy of Prof. Dr. med. Andreas von Deimling, Department of Neuropathology, University Heidelberg, Germany).
IDH mutations in other tumors
IDH1 and IDH2 mutations are almost always glioma specific. Although in most other human tumors IDH1/2 mutations are extremely rare, they have been reported at lower frequency in mesenchymal tumors, particularly haematopoietic malignancies and chondroid neoplasms. IDH mutations were identified in approximately 8% of patients with acute myeloid leukemia (AML), in myeloproliferative neoplasms and myelodysplastic syndromes. Among AML patients mutations typically affect the IDH2 gene. Rare instances of AML patients with mutations in both IDH1 and IDH2 have been described, unlike gliomas. Besides, IDH1 mutations have also been reported in endochondromas, in central or differentiated chondrosarcomas, but not in peripheral chondrosarcomas or osteochondromas.
Genetics
In addition to IDH1 and IDH2, 3 more genes have been identified (IDH3A, IDH3B. IDH3G), but have not been implicated to date in gliomas. Mutations typically affect the enzyme’s active site at codon R132 in the IDH1 gene and its homologous R172 in the IDH2 gene. Over 90% of the reported mutations affect the IDH1 gene: Interestingly, the R132H substitution accounts >90%. R132C, R132G, R132L mutations show much lower frequency (<3,2%).
Function of IDH
The IDH1 and IDH2 isoforms are structurally related with 70% of sequence identity and function as NADP+ dependent homodimers. Isocitrate dehydrogenases (IDH) are key enzymes catalyzing the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG) in the Krebs cycle, with production of NADH/NADPH. The enzymatic activity of IDH mutant proteins is significantly reduced and results in an 100-fold increase in the amount of D-2-HG. D-2-HG is structurally similar to α-KG. It functions as a competitive inhibitor of multiple α-KG-dependent enzymes, which are mainly dioxygenases with important roles for cancer. These use α-KG as co-substrate to catalyze different reactions affecting cancer, e.g. DNA-repair and response to hypoxia.
Clinical features
The mean age of brain tumor patients with IDH mutations is significantly lower in comparison with patients without IDH mutations. Moreover, the average time from the first clinical symptom to the histological diagnosis of glioma is significantly longer in patients with IDH mutations than in wild-type patients, along with a slower growth and less aggressive tumor. IDH mutations are significantly and inversely associated with the histological malignancy grade in adult patients.
Assessment of IDH mutation status
Practical guidelines for a reliable detection of IDH mutations in glioma (National Comprehensive Cancer Network (NCCN), EURO-CNS research committee) recommend to first perform immunohistochemical testing with the anti-IDH1 R132H antibody, then to follow up with DNA/PCR-based detection techniques for other less frequent IDH mutations only when the results from immunohistochemistry are negative.