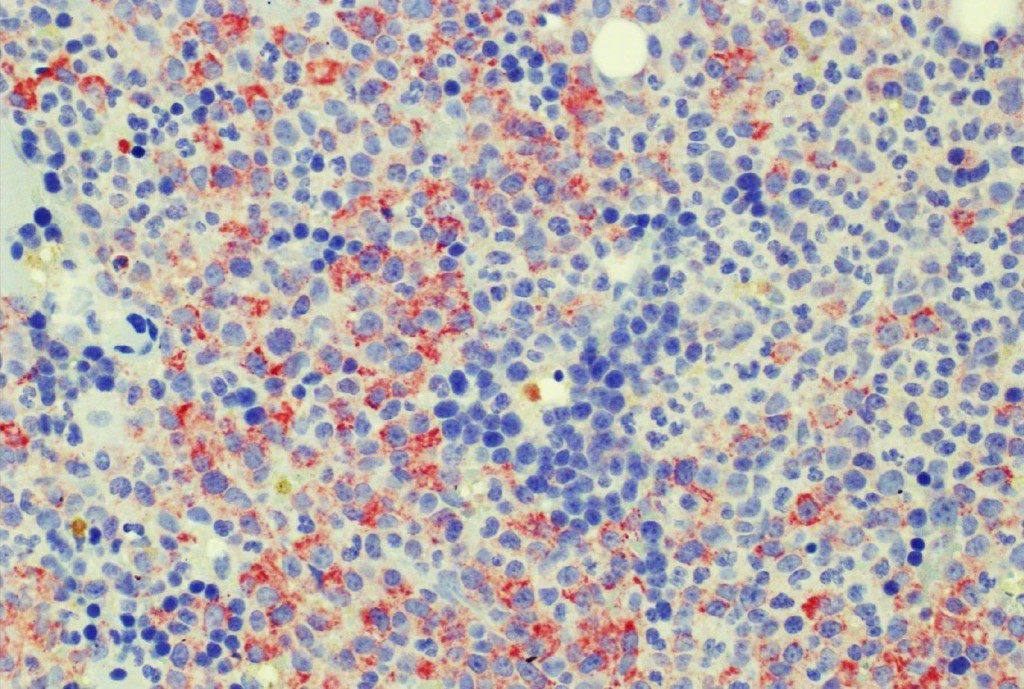
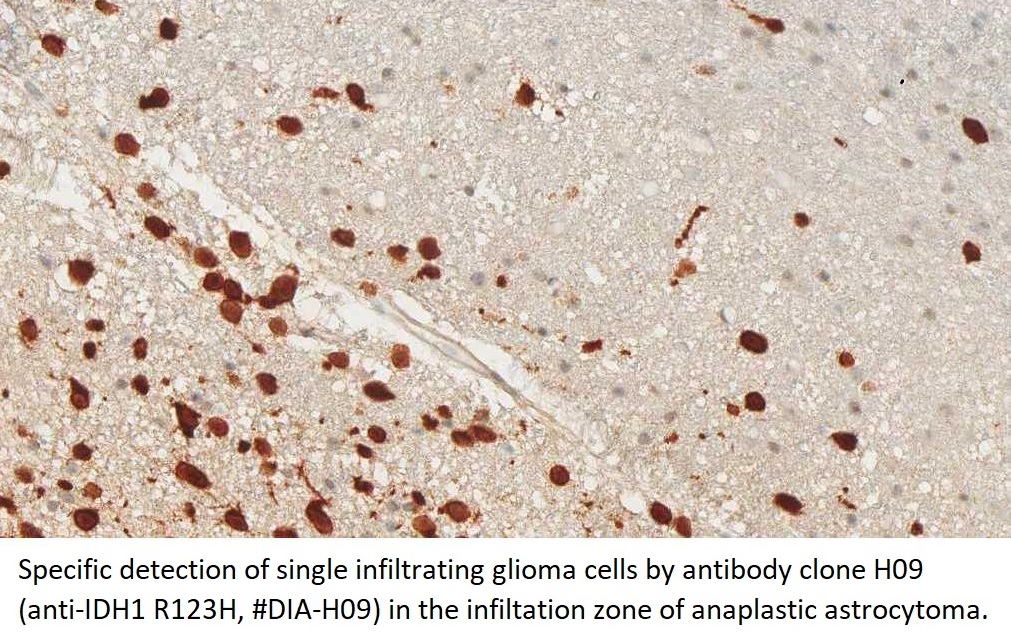
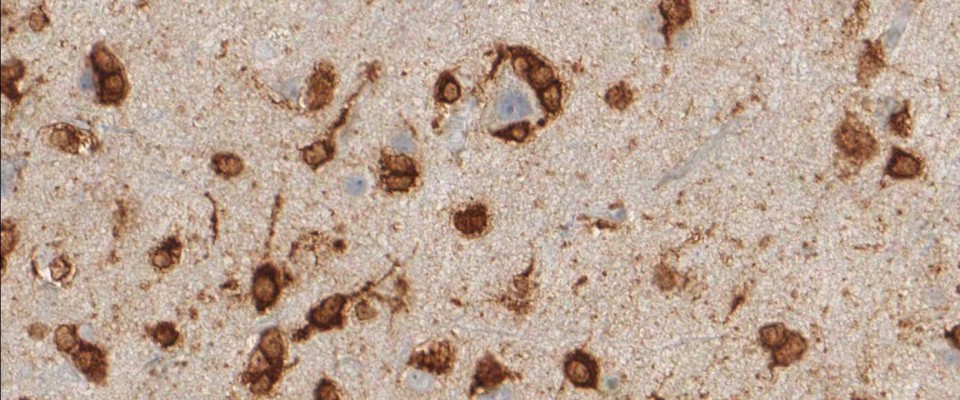
The availability of anti-IDH1 R132H antibody clone H09 (#DIA-H09, dianova, Germany) developed by the German Cancer Research Center (DKFZ) in Heidelberg has triggered fundamental advances for the 2016 WHO classification of CNS tumors (link: https://pubmed.ncbi.nlm.nih.gov/27157931/ ). IDH1 R132H IHC has been introduced as a backbone for differential diagnosis of glioma. Now, early in 2021 in the Journal Nature, researchers from Heidelberg & Mannheim report for the first time promising effects of a IDH1 R132H mutation-specific vaccine in patients with malignant brain tumors. The vaccine triggered the desired immune response in the tumors and proved to be safe.
In their recenty published study (Platten, M., Bunse, L., Wick, A. et al., Nature 2021), authors describe a multicentre, single-arm, open-label, first-in-humans phase I trial that were carried out in 33 patients with newly diagnosed World Health Organization grade 3 and 4 IDH1(R132H)+ astrocytomas. The DKFZ announces “Promising preclinical results” in their press release from March 2021. A phase II study to examine whether the IDH1 vaccine leads to better treatment results than the standard treatment alone is beeing prepared.
Up to now, theraphy had usually only limited success in halting tumor progression in patients with diffuse gliomas characterised by an IDH1 mutation, which can be identified by antibody clone H09 (#DIA-H09, dianova). We believe that the IDH1 R132H vaccine offers the potential for developing a treatment that can suppress these tumors more effectively and on a long-term basis,” commented study co-director Wolfgang Wick, Medical Director of the Neurological Clinic of Heidelberg University Hospital and Head of Division at DKFZ.
Reference:
Platten, M., Bunse, L., Wick, A. et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature (2021). https://doi.org/10.1038/s41586-021-03363-z

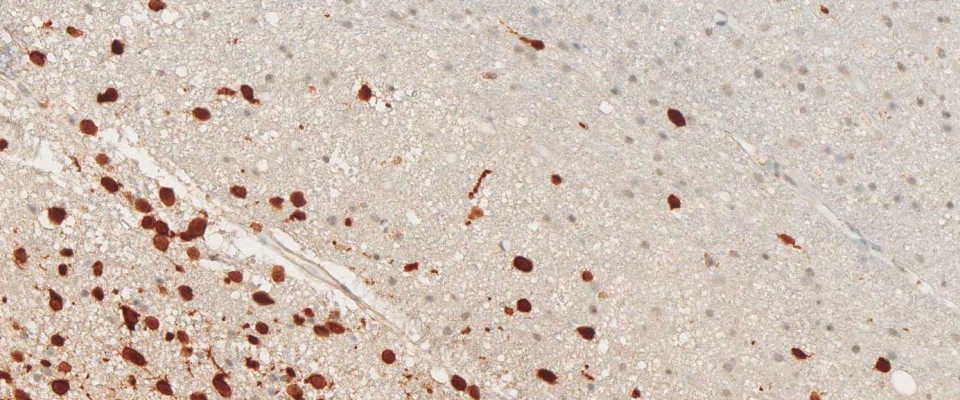
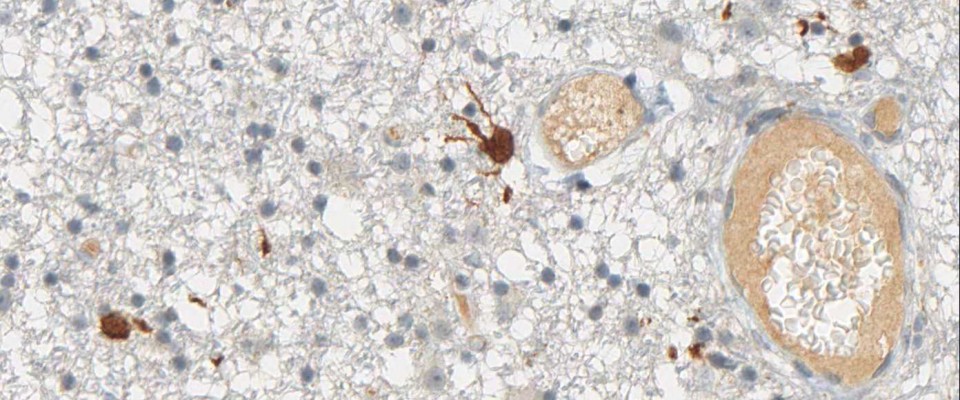
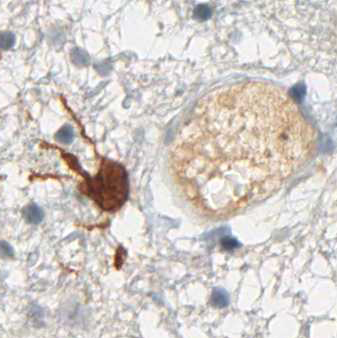

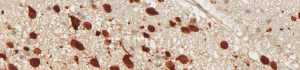
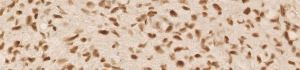 anti- ATRX clone AX1
anti- ATRX clone AX1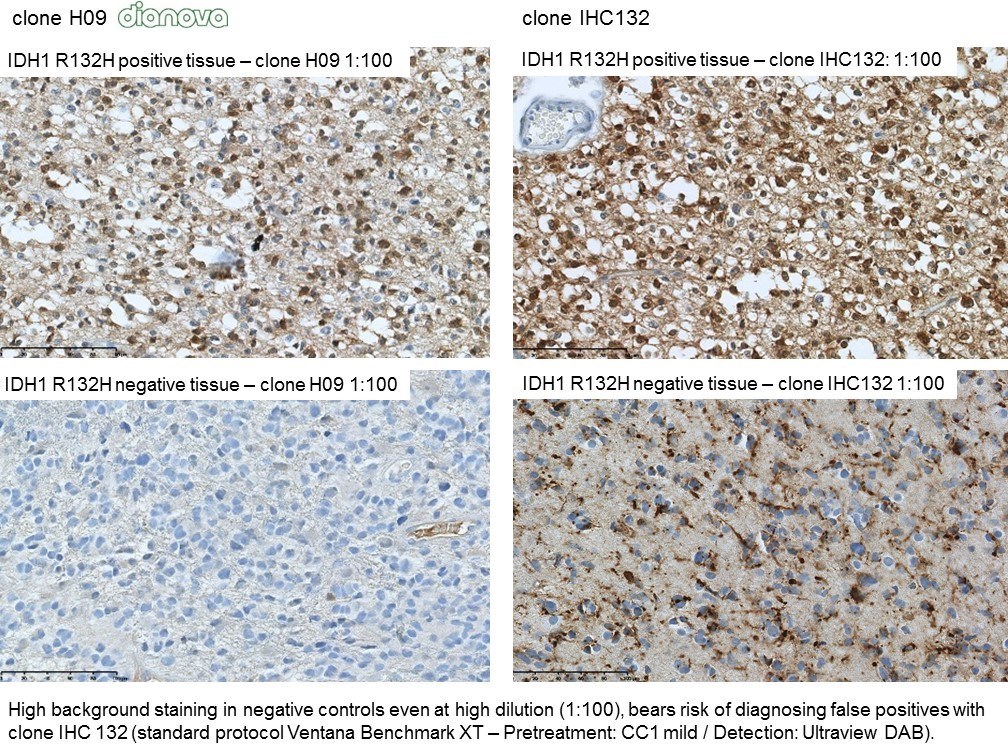
 For the first time, the WHO (World Health Organization) Classification of Tumors of the Central Nervous System uses molecular and histological analysis together to define brain tumor entities. The new concept for how CNS tumor diagnosis should be structured, moves the IDH status into focus. The algorithm for classification of the diffuse gliomas often proceeds from IDH1 R132H histology first to molecular genetic features next.
For the first time, the WHO (World Health Organization) Classification of Tumors of the Central Nervous System uses molecular and histological analysis together to define brain tumor entities. The new concept for how CNS tumor diagnosis should be structured, moves the IDH status into focus. The algorithm for classification of the diffuse gliomas often proceeds from IDH1 R132H histology first to molecular genetic features next.